In the United States, heart surgery patients have a better chance of surviving than before. Each surgeries need to be done in a carefully monitored environments, and heart surgeries are no exception.
Many hospitals use heater-cooler devices to keep patients at the right temperature during surgery. But problems with these devices can still occur, sometimes leading to serious health issues or even death.
Have you had cardiac surgery in the past few years? Did you later get a heater-cooler infection diagnosis? In this blog, let’s discuss the life-threatening bacterial infection and the legal rights to proceed with the Stockert 3T lawsuit.

Want to experience our medical record review services?
Discover Your FREE Trial: Medical Record Review
Heater-Cooler Device: What is It?
Heater-cooler devices are medical tools used to control a patient’s body temperature during open-heart surgeries. In order to care for patients during surgeries, heater cooler systems are frequently required. They are crucial instruments for heart and lung operations in particular.
One of the heater-cooler systems most frequently utilized in hospitals is the Stockert 3T device. It has water tanks that help manage the temperature.
The system includes a water tank that keeps hot and cold water separate, and a unit that heats or cools the water while letting air flow in and out. The heater-cooler then sends temperature-controlled water through closed circuits to an external machine, like a heart-lung machine, without the water coming into contact with the open air.
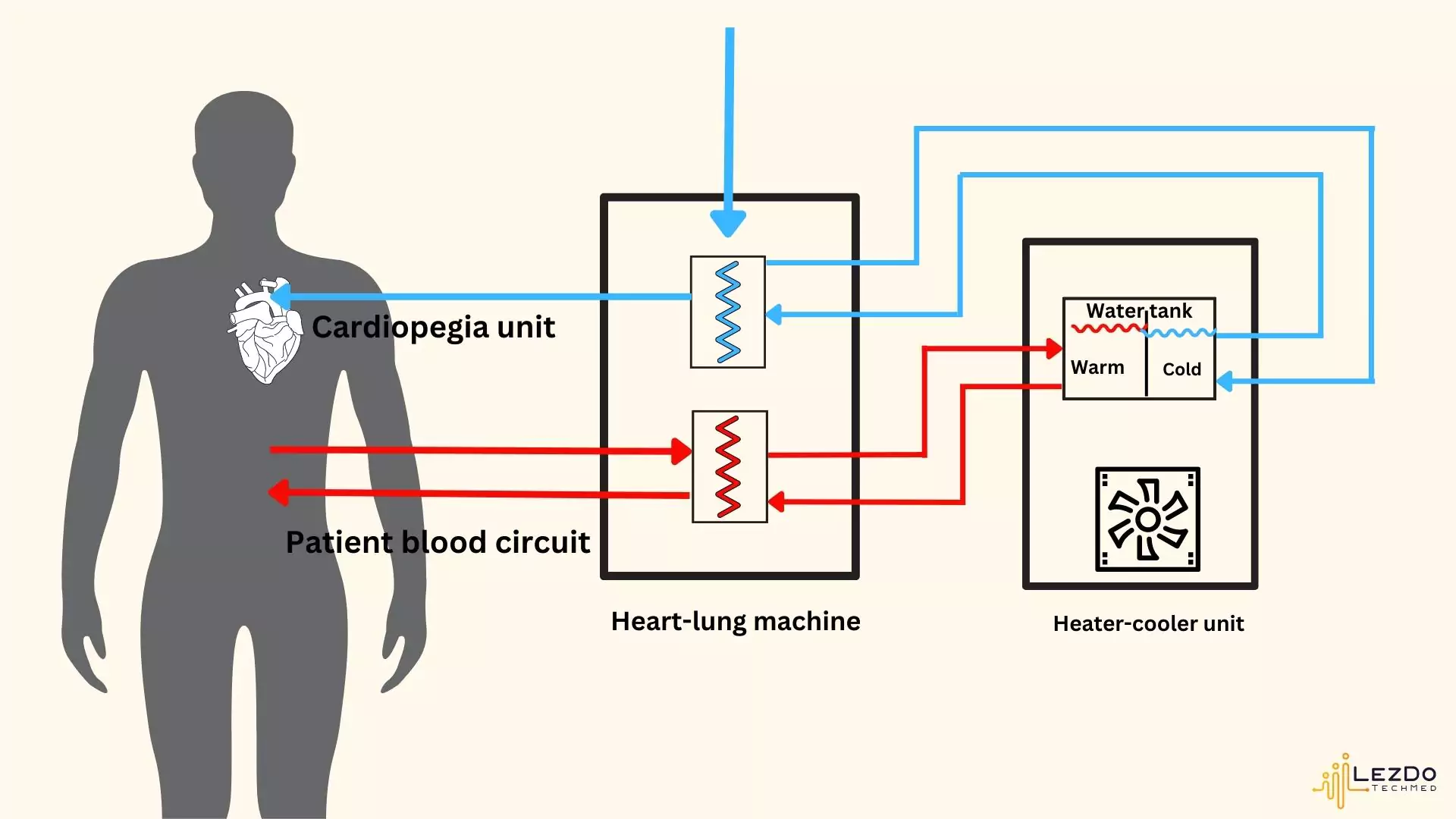
The Sorin stöckert 3t heater cooler devices, made by LivaNova, uses three circuits to keep patients warm simultaneously with the use of cold water for cardioplegia, the purposeful and brief halt of heart function during surgery.
In the United States, more than 250,000 heater-cooler heart bypass surgeries are carried out annually, and over 60% of these surgeries use devices that have been linked to heater-cooler infections.
The Sorin 3T Heater-Cooler System was the original name of the product, which was produced by the Italian company Sorin Group. In 2015, just before the initial 3T heater cooler lawsuits were brought, the business merged with Cyberonics, a corporation based in the US.
What are Nontuberculous Mycobacteria Infections?
Nontuberculous mycobacteria infections are caused by a group of bacteria found in soil, the environment, and sometimes in cardiac or medical devices contaminated with these bacteria. Mycobacterium chimaera is a slow-growing bacteria, which is one of the reasons why infection is so deadly.
Lungs are the most typical location of mycobacterium heater-cooler infection. Nontuberculous mycobacterial infections can affect the skin, soft tissue, lymph nodes, and blood, although it is uncommon for them to harm the lungs and other organs simultaneously.
According to estimates, these deadly pathogens may have infected more than 600,000 heart surgery patients countrywide since 2012.
Mycobacterium chimaera and other NTM bacteria have been linked to life-threatening infections in critically ill patients, especially those with weakened immune systems, underlying pulmonary diseases, diabetes, or those undergoing chemotherapy or other invasive medical operations, such as heart valve surgery.
The following are common heater cooler device infection symptoms:
- Fever
- Fatigue
- Pain and redness around the operation area
- Night sweats
- Muscle and joint pain
- Weight loss
- Abdominal pain
- Nausea and vomiting
It is crucial that you tell your medical professional about any potential NTM exposure you may have. Additionally, it is essential that the right testing be ordered by your doctor if they are worried about an NTM infection.
Antibiotic mixture treatments are useful for treating NTM infections. It could take longer for some patients to recover from the bacteria. Even though it is uncommon, some individuals who have had heart valve surgery may need additional surgery to halt the spread of infection within the body. If NTM infections are not identified and treated, they may become fatal.
Tired of manually reviewing medical records and searching for a reliable service to handle it for you?
Try Our Medical Record Review for FREE
What Issues does the 3T Heater-Cooler Have?
Medical equipment used during cardiac surgery called the Sorin Stockert 3T Heater-Cooler system has been connected to a potentially fatal bacterial heater-cooler infection. The Sorin 3T system is linked to an increase in mycobacterium chimaera and nontuberculous mycobacterial infections after open heart surgery.
Exposure to some heater-cooler unit cardiac surgery could cause infections in exposed patients that could manifest months to years after surgery. These units were contaminated with a rare bacteria called Mycobacterium chimaera. Mycobacterium chimaera and other bacteria can develop in the water tank of heater-cooler systems.
Even though the Stockert T3’s water never comes into contact with patients, there is still a chance that a contaminated water supply could seep into other apparatus components or spread bacteria through the air.
Air escaping the device’s exhaust vent during a surgical procedure has the potential to contaminate the patient and the surrounding area with airborne microorganisms. Aerosolization, which is the act of turning a physical substance into particles that can be carried in the air, is happening here.
Laminar flow disruption caused by the device’s exhaust fan, which is an engineering control intended to safeguard an operating room environment by decreasing airborne pollutants during surgery, is another potential factor.
After open heart surgery, patients should also be on the lookout for symptoms of endocarditis, surgical site infection, abscess, bacteremia, hepatitis, renal insufficiency, splenomegaly, pancytopenia, and osteomyelitis. These typical side effects of nontuberculous mycobacteria infections can appear up to four years following surgery.
FDA Warnings about Heater-Cooler Infection
In June 2006, the FDA in the United States gave the Stockert 3T its seal of approval. The FDA has been monitoring these devices since it has long been recognized that they have been connected to potential illnesses.
Mycobacterium chimaera contamination was discovered in testing done by the company in August 2014 on the production line and water supply at the 3T manufacturing site; however, these 3T devices were nonetheless sold all over the world.
The Food and Drug Administration (FDA) delivered a warning letter to the manufacturers on December 29, 2015, listing a number of infractions they discovered during an inspection of the facility about their production and quality management systems.
The FDA released a safety communication on Mycobacterium chimaera infections linked to Sorin 3T device use on June 1, 2016. In its first safety communication, the FDA warned that using tainted Sorin Stockert 3T heater-cooler devices could raise the risk of mycobacterial infections, which can be fatal.
Many NTM infections might not have been reported to the FDA since patients may not exhibit signs of the infection for months or years after exposure.
Regarding injuries allegedly brought on by LivaNova’s Sorin 3T contaminated heater-cooler devices, the Judicial Panel on Multidistrict Litigation consolidated roughly 40 complaints against the company in February 2018.
The FDA released a safety communication on February 25, 2020, to remind staff members and medical professionals of the precautions they should take when utilizing the LivaNova Heater-Cooler System 3T to lower the risk of infection during heart surgery.
Sorin 3T Recall
In June 2015, Sorin initiated a Class 2 recall of their 3T Heater-Cooler System, affecting 1,755 units, due to the risk of contamination that could result in NTM infections amongst patients.
Rising Heater-Cooler Infection Lawsuits
Heater-cooler infections caused by Sorin 3T gave rise to the first mycobacterium chimaera lawsuit in June 2016. During major chest procedures for the heart, lungs, and other organs, patients’ body temperatures are controlled via the company’s 3T Heater-Cooler System.
As multiple hospitals warned patients that they might have been exposed to bacterial contamination, the number quickly increased. Unaware that a bacterial infection brought on their medical issues, several patients had endured months or even years of suffering.
According to the Centers for Disease Control and Prevention, some people who contracted diseases passed away. However, it refrained from concluding that heater-cooler infections were the cause of mortality. Successful heater cooler infection lawsuits have resulted from these diseases all over the United States.
LivaNova PLC, the company that makes the Stockert 3T Heater-Cooler System, is the target of several lawsuits. Several patients who underwent cardiothoracic surgery while using a heater-cooler device and later developed infections sued the makers of those devices.
Manufacturers are required to alert the public and the FDA about potential risks when they become aware that their product may be hazardous. LivaNova PLC displayed negligence since it took too long to notify the FDA and the general public. The Stockert 3T heater-cooler has caused numerous infections and possibly wrongful deaths.
When a LivaNova heater cooler system lawsuit involves physical harm, obtaining and evaluating medical documents is a crucial step in the discovery procedure. Medical record reviews are crucial for proving prescription histories against suspected consumption in pharmaceutical mass torts.
Need High Quality Medical Record Review?
Claims against the Heater-Cooler Manufacturer
LivaNova PLC, the maker of the 3T heating and cooling system,is being sued because the device might put patients who have had heart surgery at risk of serious heater cooler infections. According to the stockert 3t lawsuits, LivaNova neglected to inform hospitals and physicians of these hazards.
Sorin 3t lawsuits claim that LivaNova created a defective product that actively encourages the growth of a fatal bacteria and exposes patients to its infection. The claim is that the manufacturing facility where these systems were created is to blame for these problems.
Lawyers for the plaintiffs argue that the company should have known that using the 3T Heater-Cooler during heart surgery could lead to infections.
Successful injury claims in this category are based on the concept of negligence. Negligence occurs when an individual, group, or employer acts carelessly and causes harm to another person. The key to winning personal injury cases is negligence.
3T Heater Cooler Lawsuit Update 2025
As per the latest update on July 2025, there are only 1 active Heater Cooler lawsuit pending in Middle district of Pennsylvania. The MDL-2816 is presided over by judge Christopher C. Conner.
Sorin 3t Settlement
LivaNova, a manufacturer of medical devices, has agreed to pay $225 million to settle almost 75% of the lawsuits brought in the US alleging that contaminated heater-cooler systems caused deadly infections during open-heart surgeries.
Two rounds of payments were made, the first ($135 million) in July 2019 and the second ($45 million) in January 2020. In conjunction with the 3T heater-cooler litigation, LivaNova set aside $294 million in its fourth quarter of 2018.
What Damages are Available in a Heater-Cooler Infection Lawsuit?
An experienced product liability attorney may also be helpful to you since they can assess the situation more thoroughly and determine the best course of action for you legally.
If you or a loved one underwent a procedure that used the Stockert 3T device and then acquired a bacterial heater-cooler infection, you might be entitled to a lawsuit. Additionally, you can be entitled to damages, which might include:
- Past and future medical expenses
- Pain and suffering
- Emotional distress
- Lost wages
- Wrongful death (If an infection brought on a loved one’s death)
The amount of compensation you receive for an accident brought on by a faulty medical device will vary depending on your injury, its circumstances, and the care and therapy you require.
To comprehend the medical difficulties involved in the case, Stockert 3T heater cooler lawyers must contact extensive medical records. LezDo TechMed’s medical record review services make it simple for lawyers to pinpoint the advantages and disadvantages of the cases they handle.
Experience Precision & Accuracy: Medical Record Review Trial
To wrap things up,
The first action you can take to protect yourself is to understand your rights. Experts believe that more people will start filing heater-cooler device lawsuits as they learn about the signs of bacterial diseases and realize they might have been exposed. The Stockert 3T heater cooler lawsuit is still active, and attorneys all over the nation are still taking new clients.
Join our growing Instagram community!
View this post on Instagram











